Chemical compounds are formed by the joining of two or more atoms. A stable compound occurs when the total energy of the combination has lower energy than the separated atoms. The bound state implies a net attractive force between the atoms ... a chemical bond. The two extreme cases of chemical bonds are:
Covalent bond: bond in which one or more pairs of electrons are shared by two atoms.
Ionic bond: bond in which one or more electrons from one atom are removed and attached to another atom, resulting in positive and negative ions which attract each other.
Other types of bonds include metallic bonds and hydrogen bonding. The attractive forces between molecules in a liquid can be characterized as van der Waals bonds.
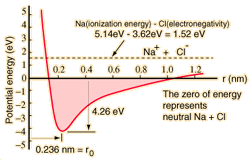
Sodium chloride
Ionic
|
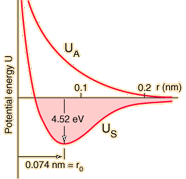
Hydrogen molecule
Covalent
Covalent Bonds
Covalent chemical bonds involve the sharing of a pair of valence electrons by two atoms, in contrast to the transfer of electrons in ionic bonds. Such bonds lead to stable molecules if they share electrons in such a way as to create a noble gas configuration for each atom.
Hydrogen gas forms the simplest covalent bond in the diatomic hydrogen molecule. The halogens such as chlorine also exist as diatomic gases by forming covalent bonds. The nitrogen and oxygen which makes up the bulk of the atmosphere also exhibits covalent bonding in forming diatomic molecules.
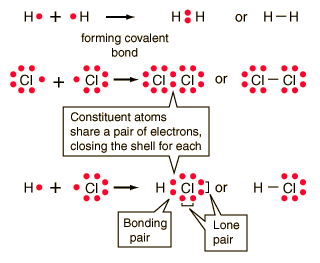 | Covalent bonding can be visualized with the aid ofLewis diagrams. |
|
Comparison of ionic and covalent materials
|
Ionic Bonds
In chemical bonds, atoms can either transfer or share their valence electrons. In the extreme case where one or more atoms lose electrons and other atoms gain them in order to produce a noble gas electron configuration, the bond is called an ionic bond.
Typical of ionic bonds are those in the alkali halides such as sodium chloride, NaCl.
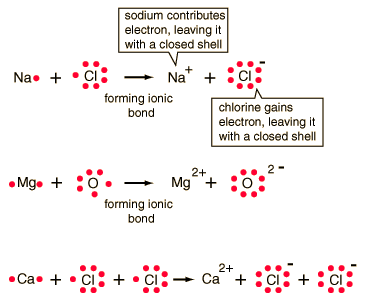 | Ionic bonding can be visualized with the aid of Lewis diagrams. |
|
Comparison of ionic and covalent materials.
|
Hydrogen Bonding
Hydrogen bonding differs from other uses of the word "bond" since it is a force of attraction between a hydrogen atom in one molecule and a small atom of high electronegativity in another molecule. That is, it is an intermolecular force, not an intramolecular force as in the common use of the word bond.
When hydrogen atoms are joined in a polar covalent bondwith a small atom of high electronegativity such as O, F or N, the partial positive charge on the hydrogen is highly concentrated because of its small size. If the hydrogen is close to another oxygen, fluorine or nitrogen in another molecule, then there is a force of attraction termed a dipole-dipole interaction. This attraction or "hydrogen bond" can have about 5% to 10% of the strength of a covalent bond.
Hydrogen bonding has a very important effect on the properties of water and ice. Hydrogen bonding is also very important in proteins and nucleic acids and therefore in life processes. The "unzipping" of DNA is a breaking of hydrogen bonds which help hold the two strands of the double helix together.
|



