There are different types of "standard" formats for straight lines; the particular "standard" format your book refers to may differ from that used in some other books. (There is, ironically, no standard definition of "standard form".) The various "standard" forms are often holdovers from a few centuries ago, when mathematicians couldn't handle very complicated equations, so they tended to obsess about the simple cases. Nowadays, you likely needn't worry too much about the "standard" forms; this lesson will only cover the more-helpful forms.
I think the most useful form of straight-line equations is the "slope-intercept" form:
- y = mx + b
I like slope-intercept form the best. It is in the form "y=", which makes it easiest to plug into, either for graphing or doing word problems. Just plug in your x-value; the equation is already solved for y. Also, this is the only format you can plug into your (nowadays obligatory) graphing calculator; you have to have a "y=" format to use a graphing utility. But the best part about the slope-intercept form is that you can read off the slope and the intercept right from the equation. This is great for graphing, and can be quite useful for word problems. C
 Speed of Construction
Speed of Construction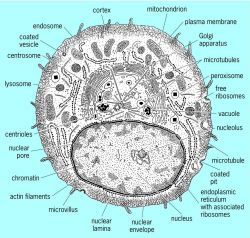



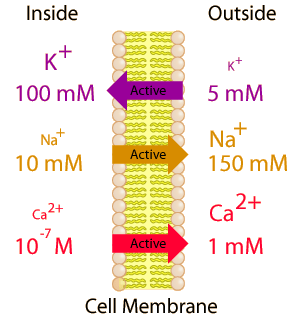
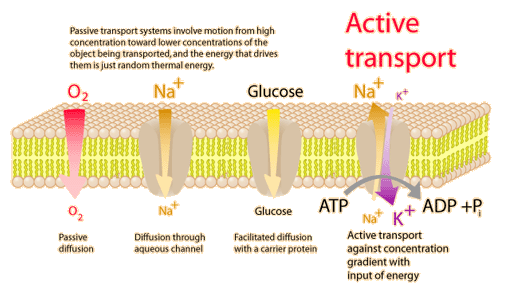
![\[
x(t) = ~~{\rm position~ at~ time~} t
\]
\[
v(t) = ~~{\rm velocity~ at~ time~} t
\]
\[
a(t) = ~~{\rm acceleration~ at~ time~} t
\]](http://www.ugrad.math.ubc.ca/coursedoc/math101/notes/applications/velocity_1.gif)
![\[
a(t) = \frac{dv}{dt}
\]](http://www.ugrad.math.ubc.ca/coursedoc/math101/notes/applications/velocity_2.gif)
![\[
\int_{T_1}^{T_2}~~a(t) ~dt= ~v(T_2)~-~v(T_1)
\]](http://www.ugrad.math.ubc.ca/coursedoc/math101/notes/applications/velocity_3.gif)
 and the final time
and the final time  , then this integral has the form
, then this integral has the form![\[
\int_{0}^{T}~~a(t) ~dt= ~v(T)~-~v(0)
\]](http://www.ugrad.math.ubc.ca/coursedoc/math101/notes/applications/velocity_6.gif)