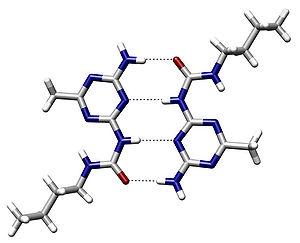
These hydrogen-bond attractions can occur between molecules (intermolecular) or within different parts of a single molecule (intramolecular).[1] The hydrogen bond (5 to 30 kJ/mole) is stronger than a van der Waals interaction, but weaker than covalent or ionic bonds. This type of bond can occur in inorganic molecules such as water and in organic molecules like DNA and proteins.
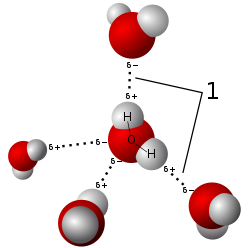
Model of hydrogen bonds (1) between molecules of water.
Contents
- 1 Bonding
- 2 History
- 3 Hydrogen bonds in water
- 3.1 Bifurcated and over-coordinated hydrogen bonds in water
- 4 Hydrogen bonds in DNA and proteins
- 5 Hydrogen bonds in polymers
Bonding
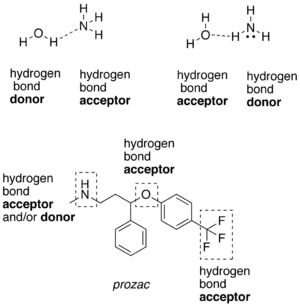
A hydrogen atom attached to a relatively electronegative atom is a hydrogen bond donor.[5] This electronegative atom is usually fluorine, oxygen, or nitrogen. An electronegative atom such as fluorine, oxygen, or nitrogen is a hydrogen bond acceptor, whether it is bonded to a hydrogen atom or not. An example of a hydrogen bond donor is ethanol, which has a hydrogen bonded to an oxygen. An example of a hydrogen bond acceptor that does not have a hydrogen atom bonded to it, is the oxygen atom in diethyl ether.
A hydrogen attached to carbon can also participate in hydrogen bonding when the carbon atom is bound to electronegative atoms, as is the case in chloroform, CHCl3.[6][7] The electronegative atom attracts the electron cloud from around the hydrogen nucleus and, by decentralizing the cloud, leaves the atom with a positive partial charge. Because of the small size of hydrogen relative to other atoms and molecules, the resulting charge, though only partial, represents a large charge density. A hydrogen bond results when this strong positive charge density attracts a lone pair of electrons on another heteroatom, which becomes the hydrogen-bond acceptor.
The hydrogen bond is often described as an electrostatic dipole-dipole interaction. However, it also has some features of covalent bonding: it is directional and strong, produces interatomic distances shorter than sum of van der Waals radii, and usually involves a limited number of interaction partners, which can be interpreted as a type of valence. These covalent features are more substantial when acceptors bind hydrogens from more electronegative donors.
The partially covalent nature of a hydrogen bond raises the following questions: "To which molecule or atom does the hydrogen nucleus belong?" and "Which should be labeled 'donor' and which 'acceptor'?" Usually, this is simple to determine on the basis of interatomic distances in the X−H…Y system: X−H distance is typically ≈110 pm, whereas H…Y distance is ≈160 to 200 pm. Liquids that display hydrogen bonding are called associated liquids.
Hydrogen bonds can vary in strength from very weak (1–2 kJ mol−1) to extremely strong (161.5 kJ mol−1 in the ion HF−
2).[8][9] Typical enthalpies in vapor include:
- F−H…:F (161.5 kJ/mol or 38.6 kcal/mol)
- O−H…:N (29 kJ/mol or 6.9 kcal/mol)
- O−H…:O (21 kJ/mol or 5.0 kcal/mol)
- N−H…:N (13 kJ/mol or 3.1 kcal/mol)
- N−H…:O (8 kJ/mol or 1.9 kcal/mol)
- HO−H…:OH+
3 (18 kJ/mol[10] or 4.3 kcal/mol; data obtained using molecular dynamics as detailed in the reference and should be compared to 7.9 kJ/mol for bulk water, obtained using the same molecular dynamics.)Hydrogen bonds in water
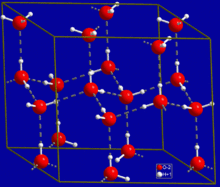
The most ubiquitous and perhaps simplest example of a hydrogen bond is found between water molecules. In a discrete water molecule, there are two hydrogen atoms and one oxygen atom. Two molecules of water can form a hydrogen bond between them; the simplest case, when only two molecules are present, is called the water dimer and is often used as a model system. When more molecules are present, as is the case with liquid water, more bonds are possible because the oxygen of one water molecule has two lone pairs of electrons, each of which can form a hydrogen bond with a hydrogen on another water molecule. This can repeat such that every water molecule is H-bonded with up to four other molecules, as shown in the figure (two through its two lone pairs, and two through its two hydrogen atoms). Hydrogen bonding strongly affects the crystal structure of ice, helping to create an open hexagonal lattice. The density of ice is less than the density of water at the same temperature; thus, the solid phase of water floats on the liquid, unlike most other substances.
Liquid water's high boiling point is due to the high number of hydrogen bonds each molecule can form, relative to its low molecular mass. Owing to the difficulty of breaking these bonds, water has a very high boiling point, melting point, and viscosity compared to otherwise similar liquids not conjoined by hydrogen bonds. Water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. For example, hydrogen fluoride—which has three lone pairs on the F atom but only one H atom—can form only two bonds; (ammonia has the opposite problem: three hydrogen atoms but only one lone pair).
- H−F…H−F…H−F
Hydrogen bonds in DNA and proteins
Hydrogen bonding also plays an important role in determining the three-dimensional structures adopted by proteins and nucleic bases. In these macromolecules, bonding between parts of the same macromolecule cause it to fold into a specific shape, which helps determine the molecule's physiological or biochemical role. For example, the double helical structure of DNA is due largely to hydrogen bonding between its base pairs (as well as pi stacking interactions), which link one complementary strand to the other and enable replication.
In the secondary structure of proteins, hydrogen bonds form between the backbone oxygens and amide hydrogens. When the spacing of the amino acid residues participating in a hydrogen bond occurs regularly between positions i and i + 4, an alpha helix is formed. When the spacing is less, between positions i and i + 3, then a 310 helix is formed. When two strands are joined by hydrogen bonds involving alternating residues on each participating strand, a beta sheet is formed. Hydrogen bonds also play a part in forming the tertiary structure of protein through interaction of R-groups.
- H−F…H−F…H−F