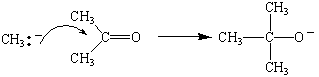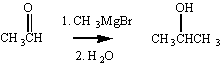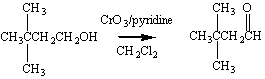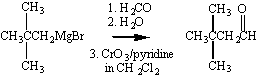Grignard Reagents
So far, we have built a small repertoire of reactions that can
be used to convert one functional group to another. We have
briefly discussed converting alkenes to alkanes; alkanes to alkyl
halides; alkyl halides to alcohols; alcohols to ethers,
aldehydes, or ketones; and aldehydes to carboxylic acids. We have
also shown how carboxylic acids can be converted into esters and
amides. We have yet to encounter a reaction, however, that
addresses a basic question: How do we make C

C bonds? One
answer resulted from the work that Francois Auguste Victor
Grignard started as part of his Ph.D. research at the turn of the
century.
Grignard noted that alkyl halides react with magnesium metal
in diethyl ether (Et
2O) to form compounds that contain
a metal-carbon bond. Methyl bromide, for example, forms
methylmagnesium bromide.
|
Et2O |
|
| CH3Br + Mg |
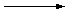 |
CH3MgBr |
Because carbon is considerably more electronegative than
magnesium, the metal-carbon bond in this compound has a
significant amount of ionic character.
Grignard reagents
such as CH
3MgBr are best thought of as hybrids of
ionic and covalent Lewis structures.
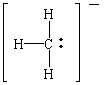
Grignard reagents are our first source of
carbanions
(literally, "anions of carbon"). The Lewis structure of
the CH
3- ion suggests that carbanions can
be Lewis bases, or electron-pair donors.
Grignard reagents such as methylmagnesium bromide are
therefore sources of a nucleophile that can attack the
+
end of the C=O double bond in aldehydes and ketones.
If we treat the product of this reaction with water, we get an
tertiary alcohol.
If we wanted to make a secondary alcohol, we could add the
Grignard reagent to an aldehyde, instead of a ketone.
By reacting a Grignard reagent with formaldehyde we can add a
single carbon atom to form a primary alcohol.
This alcohol can then be oxidized to the corresponding
aldehyde.
The Grignard reagent therefore provides us with a way of
performing the following overall transformation.
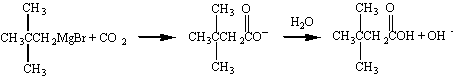
A single carbon atom can also be added if the Grignard reagent
is allowed to react with CO
2 to form a carboxylic
acid.
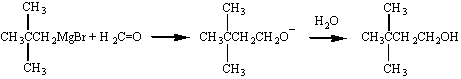
Perhaps the most important aspect of the chemistry of Grignard
reagents is the ease with which this reaction allows us to couple
alkyl chains. Isopropylmagnesium bromide, for example, can be
used to graft an isopropyl group onto the hydrocarbon chain of an
appropriate ketone, as shown in the figure below.