The physical properties of melting point, boiling point, vapor pressure,
evaporation, viscosity, surface tension, and solubility are related to
the strength of attractive forces between molecules. These attractive
forces are called
Intermolecular Forces. The amount of "stick togetherness" is important in the interpretation of the various properties listed above.
Table of Contents
- 1. Introduction
- 2. Ionic Forces
- 3. Dipole Forces
- 4. Hydrogen Bonding
- 5. Induced Dipole Forces
- 6. Contributors
Introduction
There are four types of intermolecular forces. Most of the intermolecular forces are identical to bonding between atoms in a single
molecule. Intermolecular forces just extend the thinking to forces
between molecules and follows the patterns already set by the bonding
within molecules.
Ionic Forces
The forces holding ions together in ionic solids are electrostatic
forces. Opposite charges attract each other. These are the strongest
intermolecular forces. Ionic forces hold many ions in a crystal lattice
structure. Review - Ionic Bonds
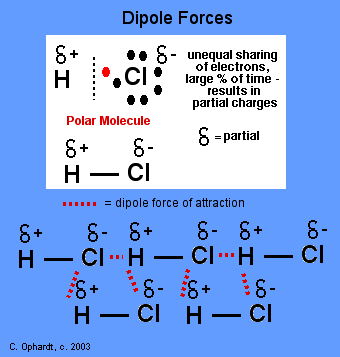
Dipole Forces
Polar
covalent molecules are sometimes described as "dipoles", meaning that
the molecule has two "poles". One end (pole) of the molecule has a
partial positive charge while the other end has a partial negative
charge. The molecules will orientate themselves so that the opposite
charges attract principle operates effectively.
In
the example on the left, hydrochloric acid is a polar molecule with the
partial positive charge on the hydrogen and the partial negative charge
on the chlorine. A network of partial + and - charges attract molecules
to each other. Review - Polar Bonds
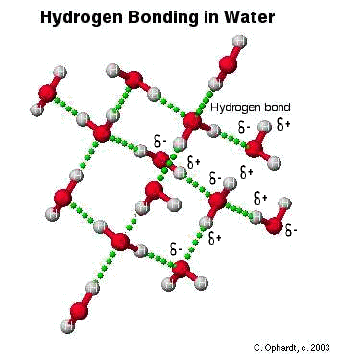
Hydrogen Bonding
The hydrogen bond
is really a special case of dipole forces. A hydrogen bond is the
attractive force between the hydrogen attached to an electronegative
atom of one molecule and an electronegative atom of a different
molecule. Usually the electronegative atom is oxygen, nitrogen, or
fluorine. In other words - The hydrogen on one molecule attached to O or
N that is attracted to an O or N of a different molecule.
In
the graphic below, the hydrogen is partially positive and attracted to
the partially negative charge on the oxygen or nitrogen. Because oxygen
has two lone pairs, two different hydrogen bonds can be made to each
oxygen. This is a very specific bond as indicated. Some combinations
that are not hydrogen bonds include: hydrogen to another hydrogen or
hydrogen to a carbon.
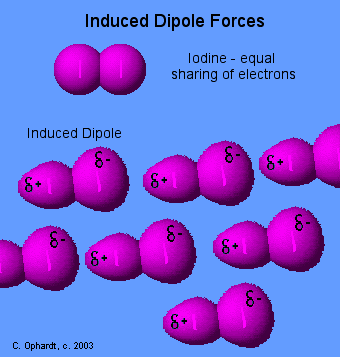
Induced Dipole Forces
Forces
between essentially non-polar molecules are the weakest of all
intermolecular forces. "Temporary dipoles" are formed by the shifting of
electron clouds within molecules. These temporary dipoles attract or
repel the electron clouds of nearby non-polar molecules.
The
temporary dipoles may exist for only a fraction of a second but a force
of attraction also exist for that fraction of time. The strength of
induced dipole forces depends on how easily electron clouds can be
distorted. Large atoms or molecules with many electrons far removed from
the nucleus are more easily distorted. Review - Non-Polar Bonds