What are intermolecular attractions?Intermolecular versus intramolecular bonds
Intermolecular attractions are attractions between one molecule and a neighbouring molecule. The forces of attraction which hold an individual molecule together (for example, the covalent bonds) are known as intramolecular attractions. These two words are so confusingly similar that it is safer to abandon one of them and never use it. The term "intramolecular" won't be used again on this site.
All molecules experience intermolecular attractions, although in some cases those attractions are very weak. Even in a gas like hydrogen, H2, if you slow the molecules down by cooling the gas, the attractions are large enough for the molecules to stick together eventually to form a liquid and then a solid.In hydrogen's case the attractions are so weak that the molecules have to be cooled to 21 K (-252°C) before the attractions are enough to condense the hydrogen as a liquid. Helium's intermolecular attractions are even weaker - the molecules won't stick together to form a liquid until the temperature drops to 4 K (-269°C).
van der Waals forces: dispersion forcesDispersion forces (one of the two types of van der Waals force we are dealing with on this page) are also known as "London forces" (named after Fritz London who first suggested how they might arise).
The origin of van der Waals dispersion forces
Temporary fluctuating dipoles
Attractions are electrical in nature. In a symmetrical molecule like hydrogen, however, there doesn't seem to be any electrical distortion to produce positive or negative parts. But that's only true on average.
The lozenge-shaped diagram represents a small symmetrical molecule - H2, perhaps, or Br2. The even shading shows that on average there is no electrical distortion.
But the electrons are mobile, and at any one instant they might find themselves towards one end of the molecule, making that end 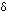 -. The other end will be temporarily short of electrons and so becomes -. The other end will be temporarily short of electrons and so becomes 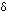 +. +. |
An instant later the electrons may well have moved up to the other end, reversing the polarity of the molecule.
This constant "sloshing around" of the electrons in the molecule causes rapidly fluctuating dipoles even in the most symmetrical molecule. It even happens in monatomic molecules - molecules of noble gases, like helium, which consist of a single atom.
If both the helium electrons happen to be on one side of the atom at the same time, the nucleus is no longer properly covered by electrons for that instant.
How temporary dipoles give rise to intermolecular attractions
I'm going to use the same lozenge-shaped diagram now to represent any molecule which could, in fact, be a much more complicated shape. Shape does matter (see below), but keeping the shape simple makes it a lot easier to both draw the diagrams and understand what is going on.
Imagine a molecule which has a temporary polarity being approached by one which happens to be entirely non-polar just at that moment. (A pretty unlikely event, but it makes the diagrams much easier to draw! In reality, one of the molecules is likely to have a greater polarity than the other at that time - and so will be the dominant one.)
As the right hand molecule approaches, its electrons will tend to be attracted by the slightly positive end of the left hand one.
This sets up an induced dipole in the approaching molecule, which is orientated in such a way that the 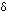 + end of one is attracted to the + end of one is attracted to the 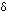 - end of the other. - end of the other.
An instant later the electrons in the left hand molecule may well have moved up the other end. In doing so, they will repel the electrons in the right hand one.
The polarity of both molecules reverses, but you still have  + attracting + attracting  -. As long as the molecules stay close to each other the polarities will continue to fluctuate in synchronisation so that the attraction is always maintained. -. As long as the molecules stay close to each other the polarities will continue to fluctuate in synchronisation so that the attraction is always maintained.
There is no reason why this has to be restricted to two molecules. As long as the molecules are close together this synchronised movement of the electrons can occur over huge numbers of molecules.
This diagram shows how a whole lattice of molecules could be held together in a solid using van der Waals dispersion forces. An instant later, of course, you would have to draw a quite different arrangement of the distribution of the electrons as they shifted around - but always in synchronisation.
The strength of dispersion forces
Dispersion forces between molecules are much weaker than the covalent bonds within molecules. It isn't possible to give any exact value, because the size of the attraction varies considerably with the size of the molecule and its shape.
|