Charging by conduction involves the contact of a charged object to a neutral object. Suppose that a positively charged aluminum plate is touched to a neutral metal sphere. The neutral metal sphere becomes charged as the result of being contacted by the charged aluminum plate. Or suppose that a negatively charged metal sphere is touched to the top plate of a neutral needle electroscope. The neutral electroscope becomes charged as the result of being contacted by the metal sphere. And finally, suppose that an uncharged physics student stands on an insulating platform and touches a negatively charged Van de Graaff generator. The neutral physics student becomes charged as the result of contact with the Van de Graaff generator. Each of these examples involves contact between a charged object and a neutral object. In contrast to induction, where the charged object is brought near but never contacted to the object being charged, conduction charging involves making the physical connection of the charged object to the neutral object. Because charging by conduction involves contact, it is often called charging by contact.
Charging by Conduction Using a Negatively Charged Object
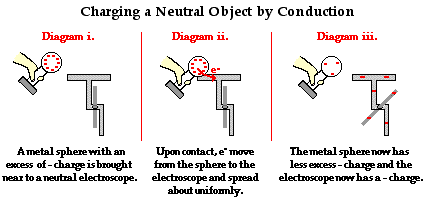
To explain the process of charging by contact, we will first consider the case of using a negatively charged metal sphere to charge a neutral needle electroscope. Understanding the process demands that you understand that like charges repel and have an intense desire to reduce their repulsions by spreading about as far as possible. A negatively charged metal sphere has an excess of electrons; those electrons find each other repulsive and distance themselves from each other as far as possible. The perimeter the sphere is the extreme to which they can go. If there was ever a conducting pathway to a more spacious piece of real estate, one could be sure that the electrons would be on that pathway to thegreener grass beyond. In human terms, electrons living in the same home despise each other and are always seeking a home of their own or at least a home with more rooms.
Given this understanding of electron-electron repulsions, it is not difficult to predict what excess electrons on the metal sphere would be inclined to do if the sphere were touched to the neutral electroscope. Once the contact of the sphere to the electroscope is made, a countless number of excess electrons from the sphere move onto the electroscope and spread about the sphere-electroscope system. In general, the object that offers the most space in which to "hang out" will be the object that houses the greatest number of excess electrons. When the process of charging by conduction is complete, the electroscope acquires an excess negative charge due to the movement of electrons onto it from the metal sphere. The metal sphere is still charged negatively, only it has less excess negative charge than it had prior to the conduction charging process.
Charging by Conduction Using a Positively Charged Object
The previous example of charging by conduction involved touching a negatively charged object to a neutral object. Upon contact, electrons moved from the negatively charged object onto the neutral object. When finished, both objects were negatively charged. But what happens if a positively charged object is touched to a neutral object? To investigate this question, we consider the case of a positively charged aluminum plate being used to charge a neutral metal sphere by the process of conduction.
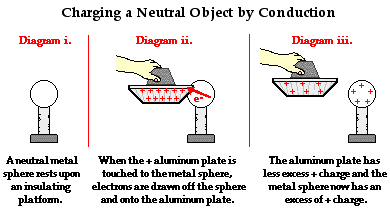
The diagram below depicts the use of a positively charged aluminum plate being touched to a neutral metal sphere. A positively charged aluminum plate has an excess of protons. When looked at from an electron perspective, a positively charged aluminum plate has a shortage of electrons. In human terms, we could say that each excess proton is rather discontented. It is not satisfied until it has found a negatively charged electron with which to co-habitate. However, since a proton is tightly bound in the nucleus of an atom, it is incapable of leaving an atom in search of that longed-for electron.
It can however attract a mobile electron towards itself. And if a conducting pathway is made between a collection of electrons and an excess proton, one can be certain that there is likely an electron that would be willing to take the pathway. So when the positively charged aluminum plate is touched to the neutral metal sphere, countless electrons on the metal sphere migrate towards the aluminum plate. There is a mass migration of electrons until the positive charge on the aluminum plate-metal sphere system becomes redistributed. Having lost electrons to the positively charged aluminum plate, there is a shortage of electrons on the sphere and an overall positive charge. The aluminum plate is still charged positively; only it now has less excess positive charge than it had before the charging process began.
The above explanation might raise a rather difficult question: Why would an electron on the previously neutral metal sphere desire to move off the metal sphere in the first place? The metal sphere is neutral; every electron on it must be satisfied since there is a corresponding proton present. What would possibly induce an electron to go through the effort of migrating to a different territory in order to have what it already has?
Law of Conservation of Charge
In each of the other methods of charging discussed in Lesson 2 -charging by friction and charging by induction - the law of conservation of charge was illustrated. The law of conservation of charge states that charge is always conserved. When all objects involved are considered prior to and after a given process, we notice that the total amount of charge among the objects is the same before the process starts as it is after the process ends. The same conservation law is observed during the charging by conduction process. If a negatively charged metal sphere is used to charge a neutral electroscope, the overall charge before the process begins is the same as the overall charge when the process ends. So if before the charging process begins, the metal sphere has 1000 units of negative charge and the electroscope is neutral, the overall charge of the two objects in the system is -1000 units. Perhaps during the charging process, 600 units of negative charge moved from the metal sphere to the electroscope. When the process is complete, the electroscope would have 600 units of negative charge and the metal sphere would have 400 units of negative charge (the original 1000 units minus the 600 units it transferred to the electroscope). The overall charge of the two objects in the system is still -1000 units. The overall charge before the process began is the same as the overall charge when the process is completed. Charge is neither created nor destroyed; it is simply transferred from one object to another object in the form of electrons.